2018
S. Autry, J. N. Gayton, R. C. Fortenberry, N. I. Hammer, and J. H. Delcamp, “Counter Anion Effect on the Photophysical Properties of Emissive Indolizine-Cyanine Dyes in Solution and Solid State,” Molecules, 23, 3051 (2018). DOI: 10.3390/molecules23123051
S. J. Cassidy, I. Brettell-Adams, L. E. McNamara, M. F. Smith, M. Bautista, H. Cao, M. Vasiliu, D. L. Gerlach, F. Qu, N. I. Hammer, D. A. Dixon, and P. A. Rupar, “Boranes with Ultra High Stokes Shifts Fluorescence,” Organometallics, 37, 3732–3741 (2018). DOI: 10.1021/acs.organomet.8b00460
B. Sajjadi, J. W. Broome, W.-Y. Chen, D. Mattern, N. O. Egiebor, N. Hammer, C. L. Smith, “Urea Functionalization of Ultrasound-Treated Biochar: A Feasible Strategy for Enhancing Heavy Metal Adsorption Capacity,” Ultrasonics Sonochemistry, 51, 20-30 (2018). DOI: 10.1016/j.ultsonch.2018.09.015
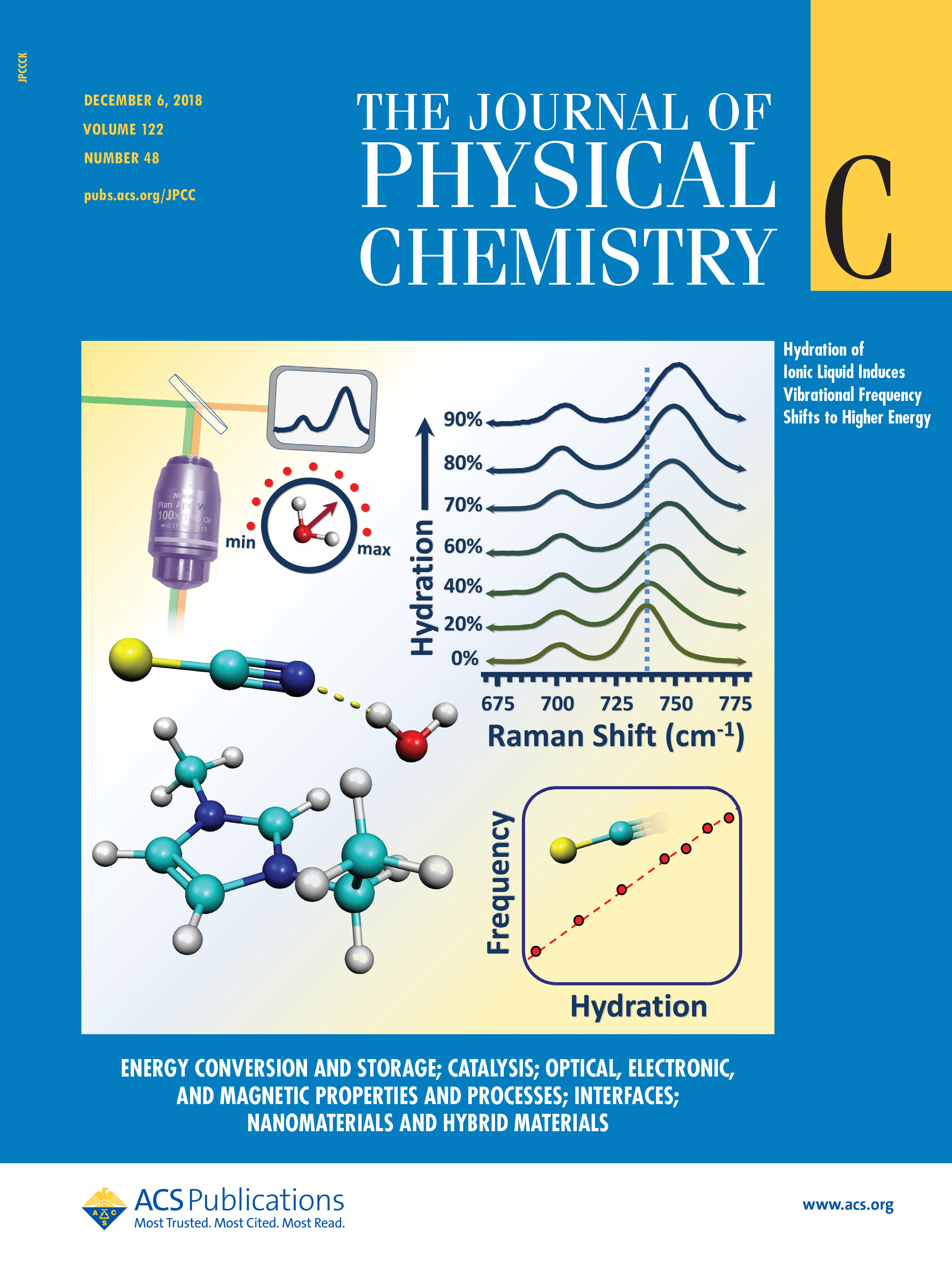
S. N. Johnson, C. R. Hutchison, C. Williams, C. L. Hussey, G. S. Tschumper and N. I. Hammer, “Intermolecular interactions and vibrational perturbations within 1-ethyl-3-methlimidazolium thiocyanate / water mixtures,” Journal of Physical Chemistry C, 122, 27673 (2018). (Featured on Cover) DOI: 10.1021/acs.jpcc.8b07114
S. G. Zetterholm, G. A. Verville, L. Boutwell, C. Boland, J. C. Prather, J. Bethea, J. Cauley, K. Warren, S. A. Smith, D. H. Magers, N. I. Hammer, “Noncovalent Interactions between Tri-methylamine N-oxide (TMAO), Urea, and Water,” Journal of Physical Chemistry B, 122, 8805 (2018). (Featured on Cover) DOI: 10.1021/acs.jpcb.8b04388
A. Peddapuram, H. Cheema, L. E. McNamara, Y. Zhang, N. I. Hammer, and J. H. Delcamp, “Quinoxaline-based Dual Donor, Dual Acceptor Organic Dyes for Dye-Sensitized Solar Cells,” Applied Sciences, 8, 1421 (2018). DOI: 10.3390/app8091421
A. O. Weldeab, A. Steen, D. J. Starkenburg, J. S. D. Williams, J. Xue, N. I. Hammer, R. K. Castellano, and D. L. Watkins, “Tuning the Structural and Spectroscopic Properties of Donor-Acceptor-Donor Oligomers via Mutual X-Bonding, H-Bonding, and π-π Interactions,” Journal of Materials Chemistry C, 6, 11992 (2018). DOI: 10.1039/C8TC00074C
N. I. Hammer and G. S. Tschumper, “Importance of a Truly Cohesive Theme in a REU Program,” in Best Practices for Chemistry REU Programs, edited by Mark Griep and Linette Watkins, ACS Books, 2018. DOI: 10.1021/bk-2018-1295.ch011
P. S. Shinde, P. Fontenot, J. P. Donahue, J. L. Waters, P. Kung, L. E. McNamara, N. I. Hammer, A. Gupta, and S. Pan, “Rapid Screening of Photoanode Materials using SPECM Technique and Formation of Z-scheme Solar Water Splitting System by Coupling p- and n-type Heterojunction Photoelectrodes,” ACS Applied Energy Materials, 1, 12283 (2018). DOI: 10.1021/acsaem.8b00381
P. S. Shinde, P. Fontenot, J. P. Donahue, J. L. Waters, P. Kung, L. E. McNamara, N. I. Hammer, A. Gupta, and S. Pan, “Synthesis and Thin Film Coating of MoS2 from [Mo3S7(S2CNEt2)3]I for Enhanced Photoelectrochemical Performance and Stability of Cu2O Photocathode Toward Efficient Solar Water Splitting,” Journal of Materials Chemistry A, 6, 9569 (2018). DOI: 10.1039/C8TA01771A
A. J. Huckaba, H. Shirley, R. W. Lamb, S. Guertin, S. Autry, H. Cheema, K. Talukdar, T. Jones, J. W. Jurss, A. Dass, N. I. Hammer, R. H. Schmehl, C. E. Webster, J. H. Delcamp, “A Mononuclear Tungsten Photocatalyst for H2 Production,” ACS Catalysis, 8, 4838 (2018). DOI: 10.1021/acscatal.7b04242
S. Nguyen, T. Ellington, K. E. Allen, J. Gorden, A. Rheingold, G. S. Tschumper, N. I. Hammer, and D. L. Watkins, “Systematic Experimental and Computational Studies of Substitution and Hybridization in Solid-State Halogen Bonded Assemblies,” ACS Crystal Growth and Design, 18, 3244 (2018). DOI: 10.1021/acs.cgd.8b00398.
Y. Zhang, H. Cheema, L. McNamara, L. A. Hunt, N. I. Hammer, J. H. Delcamp, “Ullazine Donor-Pi bridge-Acceptor Organic Dyes for DSCs,” Chemistry – A European Journal, 24, 5936 (2018). DOI: 10.1002/chem.201800030
R. Chatterjee, B. Sajjadi, D. L. Mattern, W.-Y. Chen, T. Zubatiuk, D. Leszczynska, J. Leszczynski, N. O. Egiebor, N. Hammer, “Ultrasound Cavitation Intensified Amine Functionalization: A Feasible Strategy for Enhancing CO2 Capture Capacity of Biochar,” Fuel, 225, 287 (2018). DOI: 10.1016/j.fuel.2018.03.145
C. A. Carpenter, P. Brogdon, L .E. McNamara, G. S. Tschumper, N. I. Hammer, and J. H. Delcamp, “A Robust Pyridyl-NHC Ligated Rhenium Photocatalyst for CO2 Reduction in the Presence of Water and Oxygen,” Inorganics, 6, 22 (2018). DOI:10.3390/inorganics6010022
2017
H. Cheema, A. Peddapuram, R. E. Adams, L. McNamara, L. Hunt, N. Le, D. L. Watkins, N. I. Hammer, R. H. Schmehl, and J. H. Delcamp, “Molecular Engineering of NIR Absorbing Thienopyrazine Double Donor Double Acceptor Organic Dyes for DSCs,” The Journal of Organic Chemistry, 82, 12038–12049 (2017). DOI: 10.1021/acs.joc.7b01750
L. E. McNamara, T. A. Rill, A. J. Huckaba, V. Ganeshraj, J. Gayton, R. A. Nelson, E. A. Sharpe, A. Dass, N. I. Hammer and J. H. Delcamp, “Indolizine-Squaraines: NIR Fluorescent Materials with Molecular Engineered Stokes Shifts,” Chemistry – A European Journal, 23, 12494 (2017). DOI: 10.1002/chem.201702209
K. M. Dreux, L. E. McNamara, J. T. Kelly, A. M. Wright, N. I. Hammer, and G. S. Tschumper, “Probing dative and dihydrogen bonding in ammonia borane with electronic structure computations and Raman under nitrogen spectroscopy,” Journal of Physical Chemistry A, 121, 5884 (2017). (Featured on Cover) DOI: 10.1021/acs.jpca.7b03509
J. D. Cope, J. A. Denny, R. W. Lamb, L. E. McNamara, N. I. Hammer, C. E. Webster, and T. K. Hollis, “Synthesis, Characterization, Photophysics and a Ligand Rearrangement of CCC-NHC Pincer Nickel Complexes: Colors, Polymorphs Emission and Raman Spectra,” Journal of Organometallic Chemistry, 845, 258 (2017). DOI: 10.1016/j.jorganchem.2017.05.046
Y. Zhang, S. A. Autry, L. E. McNamara, S. T. Nguyen, N. Le, P. Brogdon, D. L. Watkins, N. I. Hammer, and J. H. Delcamp, “Near-Infrared Fluorescent Thienothiadiazole Dyes with Large Stokes Shifts and High Photostability,” Journal of Organic Chemistry, 82, 5597-5606 (2017). DOI: 10.1021/acs.joc.7b00422
T. L. Ellington, P. L. Reves, B. L. Simms, J. L. Wilson, D. L. Watkins, G. S. Tschumper, and N. I. Hammer, “Quantifying the effects of halogen bonding by haloaromatic donors on the acceptor pyrimidine,” ChemPhysChem, 18, 1267–1273 (2017). (Featured on Cover) DOI: 10.1002/cphc.201700114
S. C. Sutton, W. E. Cleland, Jr., N. I. Hammer, “Introducing Students to a Synthetic and Spectroscopic Study of the Free Radical Chlorine Dioxide,” The Journal of Chemical Education, 94, 515–520 (2017). DOI: 10.1021/acs.jchemed.6b00599
K. M. Williams, M. Gruner, J. Gensheimer, A. Wright, M. Blair, S. A. Autry, and N. I. Hammer, “Partial displacement of a triamine ligand from a platinum(II) complex after reaction with N-acetylmethionine,” Inorganica Chimica Acta, 458, 163–170 (2017). DOI: 10.1016/j.ica.2017.01.010
N. N. Sreeramulu, L. McNamara, N. I. Hammer, and H. Rathnayake, “A versatile synthesis to novel binary reactive groups functionalized silsesquioxane microparticles,” Science Advances Today, 3, 25266 (2017).
J. Wang, J. Waters, P. Kung, S. Kim, J. T. Kelly, L. E. McNamara, N. I. Hammer, A. Gupta, and S. Pan, “A Facile Electrochemical Reduction Method for Improving Photocatalytic Performance of α-Fe2O3 Photoanode for Solar Water Splitting,” ACS Applied Materials and Interfaces, 9, 381-390 (2017). DOI: 10.1021/acsami.6b11057
2016
A. J. Huckaba, A. Yella, L. E. McNamara, A. E. Steen, J. S. Murphy, C. A. Carpenter, G. D. Puneky, N. I. Hammer, M. K. Nazeeruddin, M. Grätzel, and J. H. Delcamp, “Molecular Design Principles of Near-Infrared Absorbing and Emitting Indolizine Dyes,” Chemistry – A European Journal, 22, 15536-15542 (2016). DOI: 10.1002/chem.201603165
J. T. Kelly, A. K. McClellan, L. V. Joe, A. M. Wright, L. T. Lloyd, G. S. Tschumper, and N. I. Hammer, “Competition between Hydrophilic and Argyrophilic Interactions in Surface Enhanced Raman Spectroscopy (SERS),” ChemPhysChem, 17, 2782-2786 (2016). DOI: 10.1002/cphc.201600678 (Featured on Inside Cover)
S. Ananthakrishnan, J. Strain, N. N. Sreeramulu, A. Mitul, L. E. McNamara, A. Iefanova, N. I. Hammer, Q. Qiao, and H. Rathnayake, “A Novel Donor-Donor Polymeric Dyad of P3HT-block-Oligo(anthracene-9,10-diyl): Synthesis, Solid-State Packing, and Electronic Properties,” Journal of Polymer Science, Part A: Polymer Chemistry, 54, 3032-3045 (2016). DOI: 10.1002/pola.28189
N. I. Hammer, S. Sutton, J. Delcamp, and J. D. Graham, “Photocatalytic Water Splitting and Carbon Dioxide Reduction,” Handbook of Climate Change Mitigation and Adaptation, Springer, 2nd Edition, 2016.
P. Brogdon, L. E. McNamara, A. Peddapuram, N. I. Hammer, and J. H. Delcamp, “Toward Tightly Bound Carboxylic Acid-Based Organic Dyes for DSCs: Relative TiO2 Binding Strengths of Benzoic Acid, Cyanoacrylic Acid, and Conjugated Double Carboxylic Acid Anchoring Dyes,” Synthetic Metals (2016). DOI: 10.1016/j.synthmet.2016.03.031
J. Lu, K. Cuellar, N. I. Hammer, S. Jo, A. Gryczke, K. Kolter, N. Langley, M. A. Repka, “Preparation and Solid-state Characterization of Felodipine-Soluplus® Amorphous Solid Dispersions,” Drug Development and Industrial Pharmacy, 42, 485–496 (2016). DOI: 10.3109/03639045.2015.1104347
L. E. McNamara, N. Liyanage, A. Peddapuram, J. S. Murphy, J. H. Delcamp, and N. I. Hammer, “Donor-Acceptor-Donor Thienopyrazines via Pd-Catalyzed C-H Activation as NIR Fluorescent Materials,” Journal of Organic Chemistry, 81, 32–42 (2016). DOI: 10.1021/acs.joc.5b01958
J. T. Kelly, Y. Wang, X. Zhang, S. Lyapustina, M. M. Nilles, S. Xu, J. D. Graham, T. S. Tschumper, K. H. Bowen, and N. I. Hammer, “The Onset of Electron-Induced Proton-Transfer in Hydrated Azabenzene Cluster Anions,” Physical Chemistry Chemical Physics, 18, 704-712 (2016). (Featured on Cover) DOI: 10.1039/C5CP02746B
2015
J. T. Kelly, J. A. Maner, and N. I. Hammer, “Recent Advancements in Chemical Physics,” Journal of Physical Chemistry A, 119, 12909–12910 (2015). DOI: 10.1021/acs.jpca.5b12096
A. F. DeBlase, C. Wolke, G. H. Weddle, K. Archer, K. Jordan, J. T. Kelly, G. S. Tschumper, N. I. Hammer, and M. A. Johnson, “Water network-mediated, electron-induced proton transfer in anionic [C5H5N·(H2O)n]¯ clusters,” The Journal of Chemical Physics, 143, 144305 (2015). DOI: 10.1063/1.4931928
J. Wilson, J. S. D. Williams, C. Petkovsek, P. Reves, J. Jurss, N. I. Hammer, G. Tschumper, and D. L. Watkins, “Synergistic Effects of Halogen Bond and pi-pi Interactions in Thiophene-based Building Blocks,” RSC Advances, 5, 82544-82548 (2015). DOI: 10.1039/c5Ra16680b
G. E. Tyson, K. Tokmic, C. S. Oian, D. Rabinovich, H. U. Valle, T. K. Hollis, J. T. Kelly, K. A. Cuellar, L.E. McNamara, N. I. Hammer, C. E. Webster, A. G. Oliver, “Synthesis, Characterization, Photophysical Properties, and Catalytic Activity of a SCS bis(N-heterocyclic thione) (SCS-NHT) Pd Pincer Complex,” Dalton Transactions, 44, 14475 – 14482 (2015). DOI: 10.1039/C4DT03324H
J. Bae, L. E. McNamara, M. A. Nael, F. Mahdi, R. J. Doerksen, G. L. Bidwell III, N. I. Hammer and S. Jo, “Nitroreductase‐triggered activation of a novel caged fluorescent probe obtained from methylene blue,” Chemical Communications, (2015). DOI: 10.1039/C5CC03824C
N. Vunnam, N. I. Hammer, and S. Pedigo, “Basic Residue at Position 14 is Not Required for Fast Assembly and Disassembly Kinetics in Neural Cadherin,” Biochemistry, 54, 836–843 (2015). DOI: 10.1021/bi5010415
F. Begum, J. Fergusona, K. McKenna, L. E. McNamara, N. I. Hammer, and H. Rathnayake, “Preparation of n-Type Semiconducting Polymer Nanoarrays by Covalent Synthesis Followed by Crystallization,” New Journal of Chemistry, 39, 2004-2010 (2015). DOI: 10.1039/C4NJ00968A
A. J. Huckaba, F. Giordano, L. E. McNamara, K. Dreux, N. I. Hammer, G. S. Tschumper, S. M. Zakeeruddin, M. Grätzel, M. K. Nazeeruddi, and J. H. Delcamp, “Indolizine-Based Donors as Organic Sensitizer Compounds for Dye-Sensitized Solar Cells,” Advanced Energy Materials, 5, 201401629 (2015). DOI: 10.1002/aenm.201401629
2014
D. J. George and N. I. Hammer, “Studying the Binomial Distribution Using LabVIEW,” Journal of Chemical Education,92, 389–394 (2014). DOI: 10.1021/ed500684k (2014). DOI: 10.1021/ed500684k
R. N. Compton and N. I. Hammer, “Raman Under Liquid Nitrogen (RUN),” Journal of Physics: Conference Series, 548, 012017 (2014). DOI: 10.1088/1742-6596/548/1/012017
E. R. Frey, A. Sygula, N. I. Hammer, “Particle in a Disk: A Spectroscopic and Computational Laboratory Exercise Studying the Nanomolecular Building Block Corannulene,” Journal of Chemical Education, 91, 2186-2190 (2014). DOI: 10.1021/ed4005062
J. T. Kelly, S. Xu, J. Graham, J. M. Nilles, D. Radisic, A. M. Buonaugurio, K. H. Bowen, Jr., N. I. Hammer, and G. S. Tschumper, “Photoelectron Spectroscopic and Computational Study of Hydrated Pyrimidine Anions,” Journal of Physical Chemistry A,118, 11901–11907 (2014). DOI: 10.1021/jp504724v (2014). DOI: 10.1021/jp504724v
L. Xu, V. R. Manda, L. E. McNamara, M. P. Jahan, H. Rathnayake, and N. I. Hammer, “Covalent Synthesis of Perylenediimide-Bridged Silsesquioxane Nanoribbons and Their Electronic Properties,” RCS Advances, 4, 30172-30179 (2014). DOI: 10.1039/C4RA03260H
D. N. Reinemann, G. S. Tschumper, and N. I. Hammer, “Characterizing the B-P Stretching Vibration in Phosphorous Substituted Phosphine Boranes,” ChemPhysChem, 15, 1867-1871 (2014). DOI: 10.1002/cphc.201400036
K. A. Cuellar, K. L. Munroe, D. H. Magers, and N. I. Hammer, “Noncovalent Interactions in Micro-solvated Networks of Trimethylamine N-oxide,” Journal of Physical Chemistry B, 118, 449-459 (2014). DOI: 10.1021/jp408659
2013
A. M. Wright, A. A. Howard, J. C. Howard, G. S. Tschumper, and N. I. Hammer, “Charge Transfer and Blue Shifting of Vibrational Frequencies in a Hydrogen Bond Acceptor,” Journal of Physical Chemistry A, 117, 5435-5446 (2013). (cover article) DOI: 10.1021/jp401642b
A. Huckaba, B. Cao, T. K. Hollis, H. Valle, J. Kelly, N. I. Hammer, and A. Oliver, “Platinum CCC-NHC Benzimidazolyl Pincer Complexes: Synthesis, Characterization, Photostability, and Theoretical Investigations of a Blue-Green Emitter,” Dalton Transactions, 42, 8820-8826 (2013). DOI: 10.1039/c3dt50438g
H. Rathnayakea, N. Wright, A. Patel, J. Binion, L. E. McNamara, D. J. Scardino, and N. I. Hammer, “Synthesis and Characterization of Poly(3-Hexylthiophene)-Functionalized Siloxane Nanoparticles,” Nanoscale, 5, 3212-3215 (2013). DOI: 10.1039/C3NR34249B
2012
D. J. Scardino, R. Kota, D. L. Mattern, and N. I. Hammer, “Single Molecule Spectroscopic Studies of Two Organic Rectifiers,” Chemical Physics Letters, 550, 138-145 (2012).
W.-Y. Chen, G. Shi, A. K. Hailey, E. S. T. Tsai, N. I. Hammer, and Z. Wu, “Photocatalytic Conversion of Carbon Dioxide to Organic Compounds Using A Green Photocatalyst: An Undergraduate Research Experiment,” The Chemical Educator, 17, 166-171 (2012).
H. Rathnayake, J. Binion, A. McKee, D. J. Scardino, and N. I. Hammer, “Perylenediimide Functionalized Bridged-Siloxane Nanoparticles for Bulk Heterojunction Organic Photovoltaics,” Nanoscale, 4, 4631-4640 (2012).
X. Zhang, A. M. Wright, N. J. DeYonker, T. K. Hollis, N. I. Hammer, C. E. Webster, and E. Valente “Synthesis, Air-stability, Photo-bleaching, and DFT Modeling of Blue Light-Emitting Platinum CCC-N-Heterocyclic Carbene Pincer Complexes,” Organometallics, 31, 1664-1672 (2012).
J. D. Graham and N. I. Hammer, “Photocatalytic Water Splitting and Carbon Dioxide Reduction,” Handbook of Climate Change Mitigation, Springer, 2012.
2011
J. C. Howard, N. I. Hammer, and G. S. Tschumper, “Structures, Energetics and Vibrational Frequency Shifts of Hydrated Pyrimidine,” ChemPhysChem, 12, 3262-3273 (2011). DOI: 10.1002/cphc.201100457
K. L. Munroe, D. H. Magers, and N. I. Hammer, “Raman Spectroscopic Signatures of Noncovalent Interactions Between Trimethylamine N-oxide (TMAO) and Water,” Journal of Physical Chemistry B, 115, 7699-7707 (2011). DOI: 10.1021/jp203840w
D. N. Reinemann, A. M. Wright, J. D. Wolfe, G. S. Tschumper, and N. I. Hammer, “Vibrational Spectroscopy of N-Methyliminodiacetic Acid(MIDA)-Protected Boronate Ester: Assignment of the B-N Dative Bond Stretching Frequency,” Journal of Physical Chemistry A, 115, 6426-6431 (2011). DOI: 10.1021/jp112016j
D. J. Scardino, A. A. Howard, M. D. McDowell, and N. I. Hammer, “Raman Spectroscopy as the Method of Detection for Constructing a Binary Liquid-Vapor Phase Diagram,” Journal of Chemical Education, 88, 1162-1165 (2011). DOI: 10.1021/ed100016g
Q. Zhao, J. Wang, J. L. Freeman, M. Murphy-Jolly, A. M. Wright, D. J. Scardino, N. I. Hammer, C. M. Lawson, and G. M. Gray, “Syntheses, and Optical, Fluorescence and Nonlinear Optical Characterization of Phosphine-Substituted Terthiophenes,” Inorganic Chemistry, 50, 2015-2027 (2011). DOI: 10.1021/ic101624y
A. M. Wright, L. V. Joe, A. A. Howard, G. S. Tschumper, and N. I. Hammer, “Spectroscopic and Computational Insight into Weak Noncovalent Interactions in Crystalline Pyrimidine,” Chemical Physics Letters, 501, 319-323 (2011). DOI:10.1016/j.cplett.2010.11.046
D. J. Scardino, M. McDowell, J. D. Graham, and N. I. Hammer, “The Multiphoton Ionization Spectrum of Methyl Iodide Revisted: 1.67 – 2.2 eV Excitation,” Journal of Atomic and Molecular Sciences, 2, 93-98 (2011). DOI: 10.4208/jams.010511.011411a
2010
A. A. Howard, G. S. Tschumper, and N. I. Hammer, “Effects of Hydrogen Bonding on Vibrational Normal Modes of Pyrimidine,” Journal of Physical Chemistry A, 114, 6803-6810 (2010). DOI: 10.1021/jp101267w
G. S. Tschumper and N. I. Hammer, “Non-Covalent Interactions: Theory and Experiment,” Journal of the American Chemical Society, 132, 9512 (2010). DOI: 10.1021/ja104759m
M. McDowell, A. E. Wright and N. I. Hammer, “Semiconductor Nanocrystals Hybridized with Functional Ligands: New Composite Materials with Tunable Properties,” Materials, 3, 614-637 (2010). DOI: 10.3390/ma3010614
S. N. Murthy, A. B. Nair, N. I. Hammer, S. R. Kiran Vaka, and A. E. Wright, “Dermatokinetics of Nanoparticles (25 nm),” International Journal of Innovative Pharmaceutical Research, 1 (2), 37-43 (2010)
2009
A. Muraoka, Y. Inokuchi, N. I. Hammer, J.-W. Shin, M. A. Johnson, and T. Nagata, “Structural Evolution of the [(CO2)n(H2O)]- Cluster Anions: Quantifying the Effect of Hydration on the Excess Charge Accommodation Motif,” Journal of Physical Chemistry A, 113, 8942-8948 (2009). DOI: 106 3/1.2827475
M. D. Barnes, R. H. Paradise, E. Swain, D. Venkataraman, and N. I. Hammer, “Comment on Limits on Fluorescence Detected Circular Dichroism of Single Helicene Molecules,” Journal of Physical Chemistry A, 113, 9757-9758 (2009). DOI: 10.1021/jp903598t
2008
J. R. Roscioli, N. I. Hammer, M. A. Johnson, K. Diri and K. D. Jordan, “Exploring the correlation between network structure and electron binding energy in the (H2O)-7 cluster through isomer photoselected vibrational predissociation spectroscopy and ab initio calculations: Addressing complexity beyond types I-III,” Journal of Chemical Physics, 128, 104314 (2008). DOI: 10.1063/1.2827475
2007
M. Y. Odoi, N. I. Hammer, K. T. Early, K. D. McCarthy, R. Tangirala, T. Emrick and M. D. Barnes, “Fluorescence lifetimes and correlated photon statistics from single CdSe/oligo-(phenylene vinylene) composite nanostructures,” Nano Letters, 9, 2769 (2007). DOI: 10.1021/nl0713068
K. T. Early, K. D. McCarthy, N. I. Hammer, M. Y. Odoi, R. Tangirala, T. Emrick and M. D. Barnes, “Blinking suppression and intensity recurrences in single CdSe-oligo(phenylene vinylene) nanostructures: experiment and kinetic model,” Nanotechnology, 18, 424027 (2007). DOI: 10.1088/0957-4484/18/42/424027
N. I. Hammer, T. Emrick, and M. D. Barnes, “Quantum dots coordinated with conjugated organic ligands: new nanomaterials with novel photophysics,” Nanoscale Research Letters, 2, 282 (2007). (Invited Review) DOI: 10.1007/s11671-007-9062-8
M. Y. Odoi, N. I. Hammer, H. P. Rathnayake, P. M. Lahti, and M. D. Barnes, “Single Molecule Studies of a Model Fluorenone,” ChemPhysChem, 8, 1481 (2007). DOI: 10.1002/cphc.200700133
H. P. Rathnayake, A. Cirpan, F. E. Karasz, M. Y. Odoi, N. I. Hammer, M. D. Barnes, P. M. Lahti, “Luminescence of Molecular and Block Copolymeric 2,7-Bis(phenylethenyl)-fluorenones; Identifying Green-band Emitter Sites in a Fluorene-Based Luminophore,” Chemistry of Materials, 19, 3265 (2007). DOI: 10.1021/cm070552h
2006
J. R. Roscioli, N. I. Hammer, and M. A. Johnson, “Infrared Spectroscopy of Water Cluster Anions, (H2O)3-24- in the HOH Bending Region: Persistence of the Double H-Bond Acceptor (AA) Water Molecule in the Excess Electron Binding Site of the Class I Isomers,” Journal of Physical Chemistry A, 110, 7517 (2006). (Letter) DOI: 10.1021/jp062029g
N. I. Hammer, K. T. Early, M. Y. Odoi, K. Sill, T. Emrick, and M. D. Barnes, “Quantum Dot/Polymer Composites for Quantum Informatics and Sensor Applications,” Journal of Intelligence Community Research and Development (2006).
R. Hassey, E. Swain, N. I. Hammer, D. Venkataraman, and M. D. Barnes, “Probing the Chiroptical Response of a Single Molecule,” Science, 314, 1437 (2006). DOI: 10.1126/science.1134231
N. I. Hammer, K. T. Early, K. Sill, M. Y. Odoi, T. Emrick, and M. D. Barnes, “Coverage-mediated suppression of blinking in solid state quantum dot-conjugated organic composite nanostructures,” Journal of Physical Chemistry B, 110, 14167 (2006). (Cover Article) DOI: 10.1021/jp0620645f
M. Y. Odoi, N. I. Hammer, K. Sill, T. Emrick, and M. D. Barnes, “Observation of enhanced energy transfer in individual quantum dot-oligophenylene vinylene nanostructures,” Journal of the American Chemical Society, 128, 3506 (2006). (Communication) DOI: 10.1021/ja058429j
2005
N. I. Hammer, J. R. Roscioli, J. C. Bopp, J. M. Headrick, and M. A. Johnson, “Vibrational predissociation spectroscopy of the (H2O)-6-21 clusters in the OH stretching region: Evolution of the excess electron binding signature into the intermediate cluster size regime,” Journal of Chemical Physics, 123, 244311 (2005). DOI: 10.1063/1.2134701
N. I. Hammer, J. R. Roscioli, M. A. Johnson, E. M. Myshakin, and K. D. Jordan, “Infrared Spectrum and Structural Assignment of the Water Trimer Anion,” Journal of Physical Chemistry A, 109, 11526 (2005). DOI: 10.1021/jp053769c
E. G. Diken, N. I. Hammer, M. A. Johnson, R. A. Christie, and K. D. Jordan, “Mid infrared characterization of the NH4+(H2O)n clusters in the neighborhood of the n = 20 magic number,” Journal of Chemical Physics, 123, 164309 (2005). DOI: 10.1063/1.2074487
N. I. Hammer, J. R. Roscioli, and M. A. Johnson, “Identification of two distinct electron binding motifs in the anionic water clusters: A vibrational spectroscopic study of the (H2O)-6 isomers,” Journal of Physical Chemistry A, 109, 7896 (2005). (Cover Article) DOI: 10.1021/jp052144b
J. M. Headrick, E. G. Diken, R. S. Walters, N. I. Hammer, R. A. Christie, J, Cui, E. M. Myshakin, M. A. Duncan, M. A. Johnson, and K. D. Jordan, “Spectral signatures of hydrated proton vibrations in water clusters,” Science, 308, 1765 (2005). DOI:10.1126/science.1113094
N. I. Hammer, E. G. Diken, J. R. Roscioli, E. M. Myshakin, K. D. Jordan, A. B. McCoy, X. Huang, S. Carter, J. M. Bowman, and M. A. Johnson, “The vibrational predissociation spectra of the H5O2+•RGn (RG = Ar, Ne) clusters: Correlation of the solvent perturbations in the free OH and shared proton transitions of the Zundel ion,” Journal of Chemical Physics, 122, 244301 (2005). DOI:10.1063/1.1927522
J.-W. Shin, N. I. Hammer, H. Schneider, A. GloB, J. M. Weber, and M. A. Johnson, “Infrared determination of core-ion switching in the (CO2)n- clusters from both the ion and solvent perspectives,” Journal of Physical Chemistry A, 109, 3146 (2005). DOI: 10.1021/jp050092k
W. D. Robertson, N. I. Hammer, J. E. Bartmess, K. Diri, K. D. Jordan, and R. N. Compton, “Negative Ions of Ethylene Sulfite,” Journal of Chemical Physics, 122, 204319 (2005). DOI: 10.1063/1.1913578
N. I. Hammer, S. G. Stepanian, L. Adamowicz, and R. N. Compton, “Isotope Effects in Dipole-Bound Anions of Acetone,” Physical Review Letters, 94, 153004, (2005). DOI: 10.1103/PhysRevLett.94.153004
2004
N. I. Hammer, J.-W. Shin, J. M. Headrick, E. G. Diken, J. R. Roscioli, G. H. Weddle, and M. A. Johnson, “How do small water clusters bind an excess electron?,” Science, 306, 675 (2004). DOI: 10.1126/science.1102792
J.-W. Shin, N. I. Hammer, J. M. Headrick, and M. A. Johnson, “Preparation and photoelectron spectrum of the “missing” (H2O)4- cluster,” Chemical Physics Letters, 399, 675 (2004). DOI: 10.1016/j.cplett.2004.10.015
E. G. Diken, N. I. Hammer, and M. A. Johnson, “Preparation and photoelectron spectrum of the glycine molecular anion: Assignment to a dipole-bound electron species with a high-dipole moment, non-zwitterionic form of the neutral core,” Journal of Chemical Physics, 120, 9899, (2004). DOI: 10.1063/1.1755196
W. Shin, N. I. Hammer, E. G. Diken, M. A. Johnson, R. S. Walters, T. D. Jaeger, M. A. Duncan, R. A. Christie, and K. D. Jordan, “Infrared signature of structural motifs associated with the H+(H2O)n, n = 6-29, clusters,” Science, 304, 1137 (2004). DOI: 10.1126/science.1096466
E. A. Price, N. I. Hammer, and M. A. Johnson, “A cluster study of Cl2- microhydration: Size-dependent competition between symmetrical H-bonding to the anion and the formation of cyclic water networks in the Cl2-•1-5(H2O) series,” Journal of Physical Chemistry A, 108, 3910 (2004). DOI: 10.1021/jp031239f
C. Desfranois, Y. Bouteiller, J. P. Schermann, D. Radisic, S. T. Stokes, K. H. Bowen, N. I. Hammer, and R. N. Compton, “Long-range electron binding to quadrupolar molecules,” Physical Review Letters, 92, 083003 (2004). DOI: 10.1103/PhysRevLett.92.083003
N. I. Hammer, R. J. Hinde, K. Diri, K. D. Jordan, D. Radisic, K. H. Bowen, and R. N. Compton, “Dipole-Bound Anions of Highly Polar Molecules: Ethylene Carbonate and Vinylene Carbonate,” Journal of Chemical Physics, 120, 685 (2004). DOI: 10.1063/1.1629669
2003
N. I. Hammer, K. Diri, K. D. Jordan, C. Desfranois, and R. N. Compton, “Dipole-Bound Anions of Carbonyl, Nitrile, and Sulfoxide Containing Molecules,” Journal of Chemical Physics, 119, 3650 (2003). DOI: 10.1063/1.1590959 Click here for experimental and calculated molecular properties
N. I. Hammer and R. N. Compton, “Effects of Electric Fields and Collisions on Highly Excited Rubidium Atoms,” European Physical Journal D, 26, 27 (2003). DOI: 10.1140/epjd/e2003-00200-0
2002
N. I. Hammer, F. Gao, R. M. Pagni, and R. N. Compton, “Charge Transfer Reactions between Chiral Rydberg Atoms and Chiral Molecules,” Journal of Chemical Physics, 117, 4299 (2002). DOI: 10.1063/1.1496760
2001
R. N. Compton and N. I. Hammer, “Multipole-Bound Molecular Anions” in Advances in Gas-Phase Ion Chemistry Volume 4, edited by N. Adams and L. Babcock, Elsevier Science, 2001, pp. 257-291.
N. I. Hammer and R. N. Compton, “Effects of dc Electric Fields on Multiphoton Ionization of Rubidium Atoms at Low and High Densities” in Tenth International Symposium on Resonance Ionization Spectroscopy Proceedings, edited by J. Parks and J. Young, American Institute of Physics, 2001 pp. 145-153. DOI: 10.1063/1.1405596