Graduate Student Ali Baumann will Present “(HCl)m(H2O)n Clusters: Investigation into the dissociation point of strong acids using ab initio methods” to the Department.
Abstract:
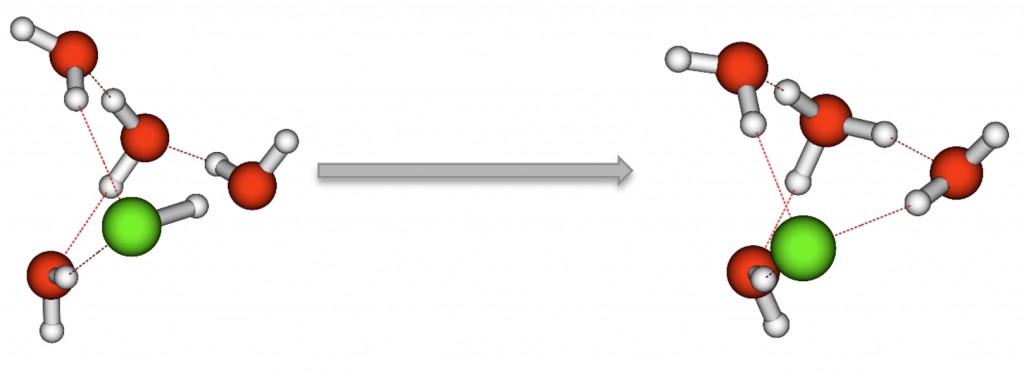
While it is known that strong acids dissociate completely in water, little is known about the mechanism through which this occurs. This is due to the fact that it is difficult to study the mechanism experimentally. Although IR spectra vibrational peaks of (HCl)m(H2O)n clusters have been produced, definitive assignment of the peaks has yet to occur. Ab initio calculations provide necessary insight into assignment of vibrational peaks for experimental data, along with insight into minimum energy structures of small clusters that are difficult to study accurately in experiment. This study examines the ionic dissociation of small (HCl)m(H2O)n clusters (m = 1-‐4, n = 1-‐ 4) using theoretical methods. Optimization and frequency calculations were performed using MP2 and CCSD(T) methods with haXZ basis sets (X = D, T, Q). All structures that were studied were minimum energy structures on the potential energy surface, with one structure from each cluster being identified as a lowest energy structure. Proton transfer was seen in four pentamer structures, where m = 1, n = 4 and m = 2, n = 3, and were also the lowest energy structures.