Join us for the American Chemical Society (ACS) Ole Miss Local Section Common Reading Experience. We will discuss The Alchemy of Air and pick up a new book to read. We will have refreshments as well. Join us!
Student Seminar: Samantha Reilly: “Spectroscopic Analyses of Factors Affecting Formation and Stability of I-Motif DNA”
 Graduate student Samantha Reilly will present her PhD research “Spectroscopic Analyses of Factors Affecting Formation and Stability of I-Motif DNA” to the department.
Graduate student Samantha Reilly will present her PhD research “Spectroscopic Analyses of Factors Affecting Formation and Stability of I-Motif DNA” to the department.
Abstract:
The four-stranded i-motif (iM) conformation of DNA has importance to a variety of biochemical systems that include nanomaterials and oncogene regulation. Fundamental studies to understand iM formation and its structure in solution still remain to be done. In this dissertation, we will discuss the use of the fluorescent base analog tC° to determine the structural state of DNA in solution. We will also demonstrate the use of tC° to determine both the hydrodynamic properties and the folding mechanism of an iM. We also determined the loop length dependence on iM formation and stability. Our hydrodynamic studies show that the iM structure has rotational correlation times similar to unfolded single-strand DNA, but displays the same structural rigidity as duplex DNA. This combination of hydrodynamic properties is unique to the iM structure. Using kinetic rates, a mechanism is proposed for the folding of a random coil oligo into the iM. The fluorescence changes in tC° describes the hydrogen bonding of the cytosines that occur during the folding of the iM. This folding mechanism shows that all hydrogen bonds form on the same time scale as the iM forms, meaning that no one base is more important than the others in forming the iM structure. The loop length dependence studies show a distinct difference in stability. The differences in thermal stability and pKa observed when lengthening loops suggests a reasonable method for gaining fine control over the thermal stability and pKa of the iM that can be readily adapted to nanomaterial usage. Our research also shows that the optimal search algorithms for finding iMs in genomic databases should be different from the algorithms currently used.
Student Seminar: Jennie Fan: “Glutamine: The Role of a Simple, Non-Essential Amino Acid in Newly Developed Methods of Tumor Imaging”
Graduate student Jennie Fan will present “Glutamine: The Role of a Simple, Non-Essential Amino Acid in Newly Developed Methods of Tumor Imaging” to the department.
Abstract:
In 1924, Otto Warburg first described the phenomena that tumor cells preferentially ran aerobic glycolysis as a source for ATP over more efficient methods like oxidative phosphorylation. Coined the Warburg Effect, his theory was consistent with experimental findings of massive glucose uptake by tumor cells. Since glycolysis 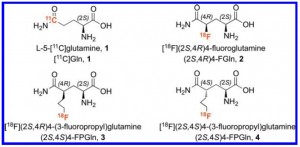 is significantly less productive than oxidative phosphorylation, tumor cells must uptake large amounts of glucose to provide enough ATP for cell growth and proliferation. This metabolic characteristic of tumor cells has been used to develop important radiotracers, like [18F]fluoro-2-deoxy-D-glucose (18-FDG), for tumor imaging. Since tumor cells are able to uptake significantly more of the glucose radiotracer, tumor imaging is simple and clear by Positron Emission Tomography (PET) scan. Although 18-FDG is currently the industry standard radiotracer for tumor imaging, use of 18-FDG can yield both false positive and false negative results for tumor indication. Several research groups propose that glutamine is another important, essential component of tumor cell growth, and research shows that attempts at targeting glutamine metabolism of cancer cells could prove beneficial. Recently, a new glutamine radiotracer, [18F](2S,4S)-4-(3- Fluoropropyl)glutamine, has surfaced and shows great promise as an effective, alternative tumor imaging agent. The advantages and disadvantages of targeting glucose versus glutamine metabolism for cancer imaging will be discussed.
is significantly less productive than oxidative phosphorylation, tumor cells must uptake large amounts of glucose to provide enough ATP for cell growth and proliferation. This metabolic characteristic of tumor cells has been used to develop important radiotracers, like [18F]fluoro-2-deoxy-D-glucose (18-FDG), for tumor imaging. Since tumor cells are able to uptake significantly more of the glucose radiotracer, tumor imaging is simple and clear by Positron Emission Tomography (PET) scan. Although 18-FDG is currently the industry standard radiotracer for tumor imaging, use of 18-FDG can yield both false positive and false negative results for tumor indication. Several research groups propose that glutamine is another important, essential component of tumor cell growth, and research shows that attempts at targeting glutamine metabolism of cancer cells could prove beneficial. Recently, a new glutamine radiotracer, [18F](2S,4S)-4-(3- Fluoropropyl)glutamine, has surfaced and shows great promise as an effective, alternative tumor imaging agent. The advantages and disadvantages of targeting glucose versus glutamine metabolism for cancer imaging will be discussed.
Heiden, Matthew G. Vander, Lewis C. Cantley, and Craig B. Thompson. “Understanding The Warburg Effect: The Metabolic Requirements Of Cell Proliferation.” Science (Washington D C) 324.5930 (n.d.): 1029-1033. Biological Abstracts.
Wise, D. R.; Thompson, C. B. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem. Sci. 2010, 35, 427−33.
Wu, Zehui, et al. “[(18)F](2S,4S)-4-(3-Fluoropropyl)Glutamine As A Tumor Imaging Agent.” Molecular Pharmaceutics 11.11 (2014): 3852-3866
Student Seminar: Thomas Ellington: “Computational Investigation into the Hydration of Sulfuric Acid”
Graduate student Thomas Ellington will present “Computational Investigation into the Hydration of Sulfuric Acid: H2SO4(H2O)n where n = 1-5″ a seminar to the department.
Abstract:
This study examines hydrated sulfuric acid clusters, H2SO4(H2O)n where n = 1-5. The structures, energetics, and thermodynamic properties of these clusters are computed. For each isomer, canonical second-order Møller-Plesset perturbation theory (MP2) was utilized along with a series of Dunning’s correlation consistent basis sets augmented with diffuse functions on all non-hydrogen atoms (heavy-aug-cc-pVXZ, where X = D, T, and Q) to determine structures and corresponding harmonic vibrational frequencies. Discrepancies in previous literature lead to uncertainties when predicting the most thermodynamically favourable hydrates1-3. To resolve this ambiguity, free energies of binding are computed to determine sequential cluster growth for nucleation. Atmospheric aerosols are known to have a significant net cooling effect on the atmosphere. Of all atmospheric aerosols, sulfates contribute most to the global radiation balance and have the ability to serve as important cloud condensation nuclei (CCN) due to their tendency to form large hydrated clusters. CCN’s, when in high concentrations, can increase cloud reflectivity and consequently will reduce the amount of solar radiation reaching the Earth’s surface.
- A.Bandy and J. Ianni. Study of the Hydrates of H2SO4 Using Density Functional Theory . J. Phys. Chem. A 1998, 102, 6533-6539.
- Theo Kurtén et al. Quantum chemical studies of hydrate formation of H2SO4 and HSO4–. Boreal Environment Research, 12:431-453, 2007.
- George C. Shields et al. Quantum Mechanical Study of Sulfuric Acid Hydration: Atmospheric Implications. J. Phys. Chem. A 2012, 116, 2209-2224.
Student Seminar: Matt Dukes: “A Proposal for The Molecular Basis of Memory”
Graduate student Matt Dukes will present “A Proposal for The Molecular Basis of Memory” to the department.
Abstract:
Despite over a century’s worth of extensive thought and experimentation, the molecular basis of biologic memory remains elusive. Recent efforts in neuroscience have attempted to explain this phenomenon in terms of a “synaptic plasticity” model, but this model fails to account for the energetic requirements, kinetics, and breadth of information encoding/decoding involved in cognition. By drawing an analogy to a computer ionic memory chip, the authors have proposed a tripartite biochemical mechanism to describe the processing of cognitive information. This model is comprised of three main components: the neuron, the surrounding neural extracellular matrix (nECM), and various trace metals (or dopants) distributed within the matrix. The authors propose that the neuron is attuned to consequences of complexation events of an extracellular moiety to a metal (formation of a cognitive unit of information, or cuinfo), such as changes in the dielectric properties, viscoelasticity, and stability of local, molecular ensembles. The supportive evidence provided for this model correlates memory loss to a deficiency or toxicity of different trace metals, loss of the means to deliver/transport metals to the matrix, or degradation of the matrix. Additionally, strengths and weaknesses of the proposed model will be discussed.
Marx and Gilon (2012). ACS Chem. Neurosci. 3, 633-642.
Marx and Gilon (2013). ACS Chem. Neurosci. 4, 983-993.
Marx and Gilon (2014). Frontiers in aging neuroscience, 6, 1-8.
Student Seminar: Katelyn Dreux: “Exploring the Potential Energy Surface of the Water∙∙∙Oxygen van der Waals complex”
Graduate student Katelyn Dreux will present “Exploring the Potential Energy Surface of the H2O∙∙∙O2 van der Waals complex” to the department.
Abstract:
Over the past two decades, numerous studies into atmospheric interactions have been conducted as a result of increasing interest in a variety of areas, including solar radiation, greenhouse gases, and cloud formation. Water and oxygen are both present and highly abundant in the troposphere, making this an important complex for study. The H2O…O2 dimer is of particular interest because of its potential viability as a greenhouse gas. Water’s role in the Earth’s radiation budget is well defined, as it can absorb IR radiation from the Earth.1 O2 is IR inactive, but upon dimerization with water, changes in frequency and intensity may occur. The intermolecular modes that result from such an association with large intensities could indicate the complex’s ability to absorb IR radiation.1 Results will be presented from a series of full geometry optimizations and harmonic vibrational frequency computations performed at the UCCSD(T) level using Dunning’s correlation consistent basis sets with diffuse functions on every atom but hydrogen (haXZ, where X = D,T,Q). Seven different structures were considered, six C2v structures, four planar and two non-planar, and one with Cs symmetry. The Cs structure has an electronic binding energy of -0.70 kcal mol-1 and is the global minimum at the UCCSD(T)/haQZ level of theory. Binding energies for the C2v structures range from -0.26 to -0.59 kcal mol-1, highlighting the very shallow nature of the potential energy surface. Analysis of the vibrational frequencies indicates that the formation of the H2O…O2 complex produces two low energy intermolecular modes under 100 cm-1 with large IR activities. A similar work studied the interaction of H2O…N2, which is more strongly bound but experiences no significantly enhanced IR activity.3
References:
1 Svishchev, I.; Boyd, R. J. Chem. Phys. A.102, 7294(1998).
2 Sabu, A.; Kondo, S.; Miura, N.; Hashimoto, K. Chem. Phys.Lett. 391, 101(2004).
3 Ellington, Thomas L.; Tschumper, Gregory S. Comput. Theor. Chem.1021,109(2013).
Student Seminar: Trey Vaughan
Graduate student Trey Vaughan will present a seminar to the department.
Abstract:
High Throughput Experimentation (HTE) has made easy a great number of the more difficult problems currently under study in chemistry. Whether the task be optimization of complex synthetic methodologies, materials synthesis, or catalyst discovery, HTE offers the capability to deconvolute large problems in a highly-efficient and rapid manner. HTE’s efficiency is due, in large part, to highly automated and precise control of experimental parameters from µM to pilot plant reactor scales. The speed at which discovery may be carried out is made possible by rapid and highly sensitive spectroscopic techniques, data management, and powerful evolutionary algorithms. At no other time in chemistry has the convergence of so many areas of expertise been so effectively integrated. Whether the expertise be analytical, synthetic, spectroscopic, or theoretical, HTE integrates all disciplines into one suite. in silico in vitro in situ, only in HTE.
Seminar: Prof. Davita Watkins: “Supramolecular Approaches towards Functional Materials”
Prof. Davita Watkins will present “Supramolecular Approaches towards Functional Materials” to the department.
Seminar: Dr. H. Lee Woodcook (University of South Florida)
Dr. Woodcook will present “Identification and characterization of non-covalent interactions that govern enzyme – substrate binding and reaction” to the department.
Abstract:
In this presentation I will highlight two biochemical processes that are governed by key intermolecular interactions. The first focuses on the initial enzymatic step of isoprenoid biosynthesis with the second highlighting binding and specificity of β-lactam anitbiotics. 1-deoxy-d-xylulose 5-phosphate synthase (DXS) is a thiamine diphosphate (TDP) dependent enzyme that marks the beginning of the non-mevalonate isoprenoid biosynthesis pathway. The mechanism of action for DXS is still poorly understood and begins with the formation of a thiazolium ylide ion. This TDP activation step is thought to proceed through an intramolecular deprotonation by the 4’-aminopyrimidine ring of TDP. The intramolecular deprotonation is catalyzed by the deprotonation of the 4’-amino group mediated by either a histidine residue or a water molecule found proximal. In the interest of gaining a better molecular understanding, QM/MM techniques were used to compute the reaction energy profiles of the proposed mechanisms. The results show a ∼10 kcal/mol difference in transition state energies favoring the water mediated mechanism. The molecular differences that led to this observed difference were probed further and the results will be presented. Bacterial resistance to standard (i.e. β-lactam-based) antibiotics has become a global pandemic. One possible key to unraveling critical details is characterization of the non-covalent interactions that govern binding and specificity (DD-peptidases, antibiotic targets, versus β-lactamases, the evolutionarily derived enzymes that play a major role in resistance) and ultimately resistance as a whole. Results of a detailed computational analysis targeted at elucidating these effects will be presented. Specifically, an extended π-π network is elucidated that suggests antibacterial resistance has evolved, in part, due to stabilizing aromatic interactions. Additionally, interactions between the protein and peptidomimetic substrate are identified and characterized; revealing an interaction that may significantly contribute to β-lactam specificity. Finally, interaction information is used to suggest modifications to current β-lactam compounds that should both improve binding and specificity in DD-peptidases and their physiochemical properties.
Seminar: Charles Campana (Bruker): “Single Crystal X-Ray Diffraction – A Tool for Synthetic Chemists”
Charles Campana from Bruker will present “Single Crystal X-Ray Diffraction – A Tool for Synthetic Chemists” to the department.


